suppressPackageStartupMessages({
library(tidyverse)
library(Seurat)
library(magrittr)
library(dplyr)
library(purrr)
library(ggplot2)
library(here)
library(runSeurat3)
library(SingleCellExperiment)
library(RColorBrewer)
library(pheatmap)
library(scater)
library(scran)
library(ggsci)
})examine adult iLN only
load packages
heatmap function
avgHeatmap <- function(seurat, selGenes, colVecIdent, colVecCond=NULL,
ordVec=NULL, gapVecR=NULL, gapVecC=NULL,cc=FALSE,
cr=FALSE, condCol=FALSE){
selGenes <- selGenes$gene
## assay data
clusterAssigned <- as.data.frame(Idents(seurat)) %>%
dplyr::mutate(cell=rownames(.))
colnames(clusterAssigned)[1] <- "ident"
seuratDat <- GetAssayData(seurat)
## genes of interest
genes <- data.frame(gene=rownames(seurat)) %>%
mutate(geneID=gsub("^.*\\.", "", gene)) %>% filter(geneID %in% selGenes)
## matrix with averaged cnts per ident
logNormExpres <- as.data.frame(t(as.matrix(
seuratDat[which(rownames(seuratDat) %in% genes$gene),])))
logNormExpres <- logNormExpres %>% dplyr::mutate(cell=rownames(.)) %>%
dplyr::left_join(.,clusterAssigned, by=c("cell")) %>%
dplyr::select(-cell) %>% dplyr::group_by(ident) %>%
dplyr::summarise_all(mean)
logNormExpresMa <- logNormExpres %>% dplyr::select(-ident) %>% as.matrix()
rownames(logNormExpresMa) <- logNormExpres$ident
logNormExpresMa <- t(logNormExpresMa)
rownames(logNormExpresMa) <- gsub("^.*?\\.","",rownames(logNormExpresMa))
## remove genes if they are all the same in all groups
ind <- apply(logNormExpresMa, 1, sd) == 0
logNormExpresMa <- logNormExpresMa[!ind,]
genes <- genes[!ind,]
## color columns according to cluster
annotation_col <- as.data.frame(gsub("(^.*?_)","",
colnames(logNormExpresMa)))%>%
dplyr::mutate(celltype=gsub("(_.*$)","",colnames(logNormExpresMa)))
colnames(annotation_col)[1] <- "col1"
annotation_col <- annotation_col %>%
dplyr::mutate(cond = gsub(".*_","",col1)) %>%
dplyr::select(cond, celltype)
rownames(annotation_col) <- colnames(logNormExpresMa)
ann_colors = list(
cond = colVecCond,
celltype=colVecIdent)
if(is.null(ann_colors$cond)){
annotation_col$cond <- NULL
}
## adjust order
logNormExpresMa <- logNormExpresMa[selGenes,]
if(is.null(ordVec)){
ordVec <- levels(seurat)
}
logNormExpresMa <- logNormExpresMa[,ordVec]
## scaled row-wise
pheatmap(logNormExpresMa, scale="row" ,treeheight_row = 0, cluster_rows = cr,
cluster_cols = cc,
color = colorRampPalette(c("#2166AC", "#F7F7F7", "#B2182B"))(50),
annotation_col = annotation_col, cellwidth=15, cellheight=10,
annotation_colors = ann_colors, gaps_row = gapVecR, gaps_col = gapVecC)
}set dir and load sample
basedir <- here()
seurat <- readRDS(paste0(basedir, "/data/AllSamplesMerged_seurat.rds"))
colLoc <- c("#61baba", "#ba6161")
names(colLoc) <- unique(seurat$location)
colAge <- c("#440154FF", "#3B528BFF", "#21908CFF", "#5DC863FF", "#FDE725FF")
names(colAge) <- c("E18" , "P7", "3w", "8w","E17to7wk")
colPal <- c("#DAF7A6", "#FFC300", "#FF5733", "#C70039", "#900C3F", "#b66e8d",
"#61a4ba", "#6178ba", "#54a87f", "#5468a8", "#25328a",
"#b6856e", "#0073C2FF", "#EFC000FF", "#868686FF", "#CD534CFF",
"#7AA6DCFF", "#003C67FF", "#8F7700FF", "#3B3B3BFF", "#A73030FF",
"#4A6990FF")[1:length(unique(seurat$RNA_snn_res.0.25))]
names(colPal) <- unique(seurat$RNA_snn_res.0.25)
colCond <- c("#446a7f", "#cb7457")
names(colCond) <- c("LTbR", "WT")
colDat <- colDat <- c(pal_npg()(10),pal_futurama()(12), pal_aaas()(10),
pal_jama()(8))[1:length(unique(seurat$dataset))]
names(colDat) <- unique(seurat$dataset)DimPlot all
clustering
DimPlot(seurat, reduction = "umap", group.by = "RNA_snn_res.0.25",
cols = colPal, raster = F, shuffle =T)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")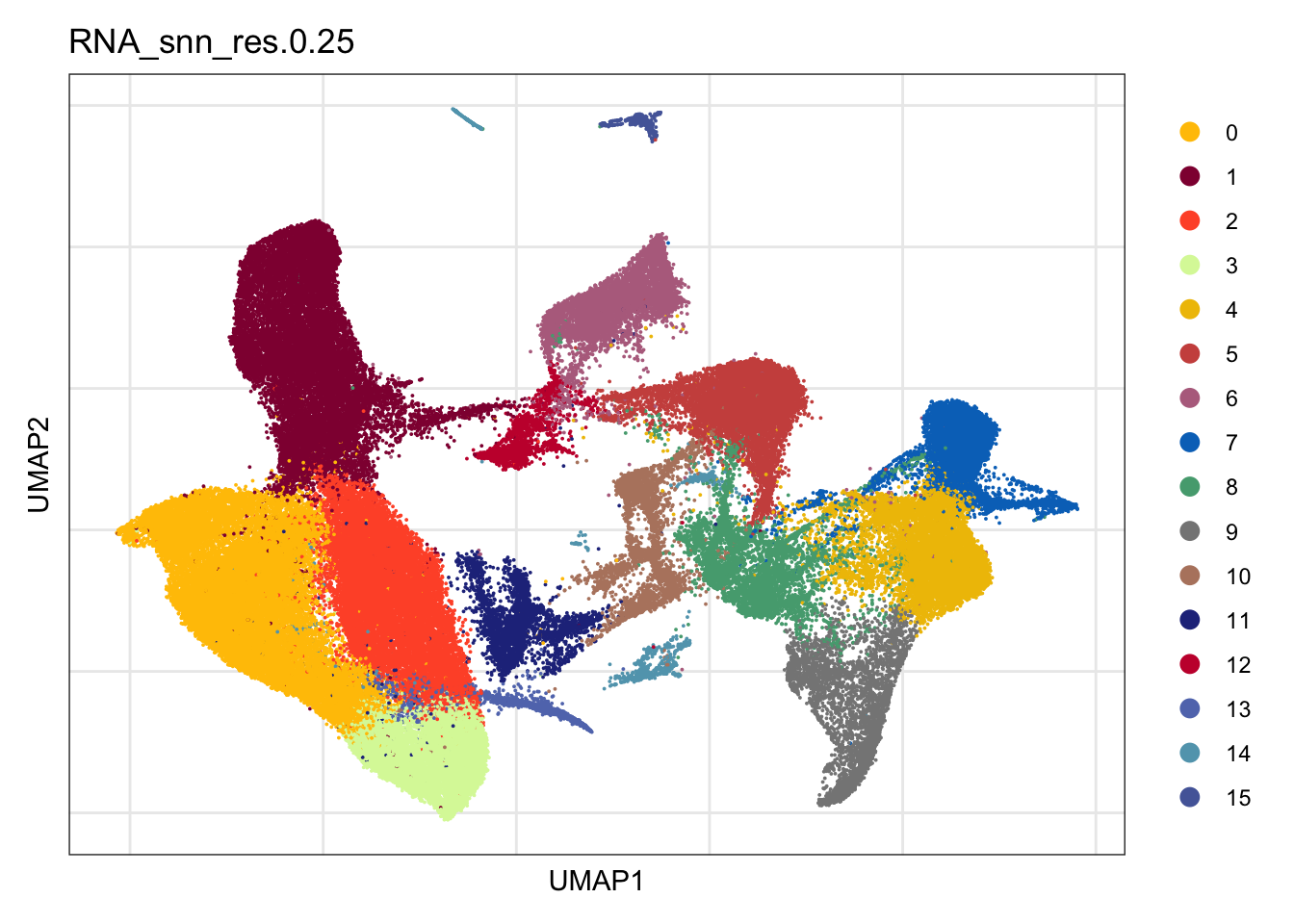
timepoint
DimPlot(seurat, reduction = "umap", group.by = "age", cols = colAge,
raster = F, shuffle =T)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")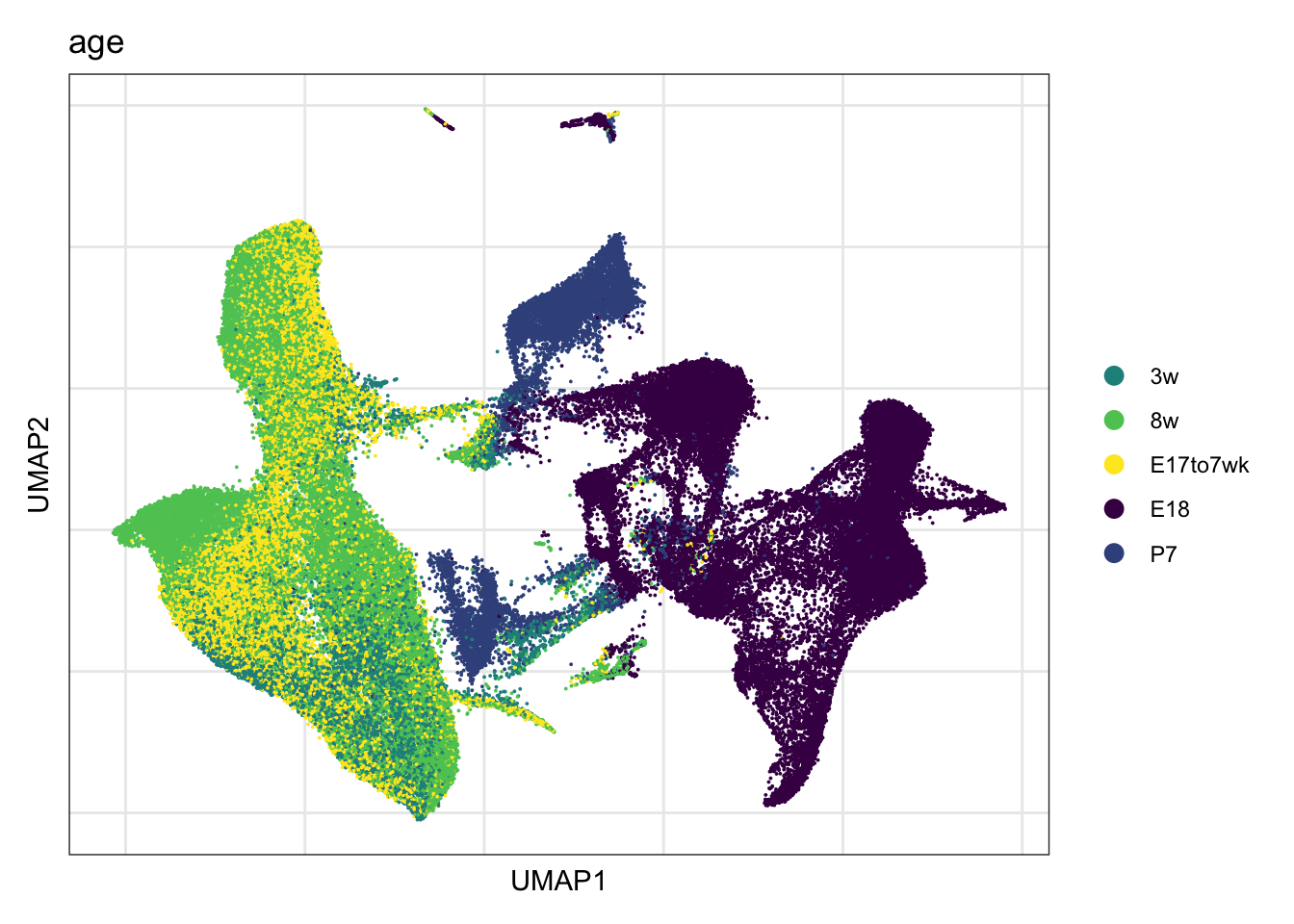
location
DimPlot(seurat, reduction = "umap", group.by = "location", cols = colLoc,
raster = F, shuffle =T)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")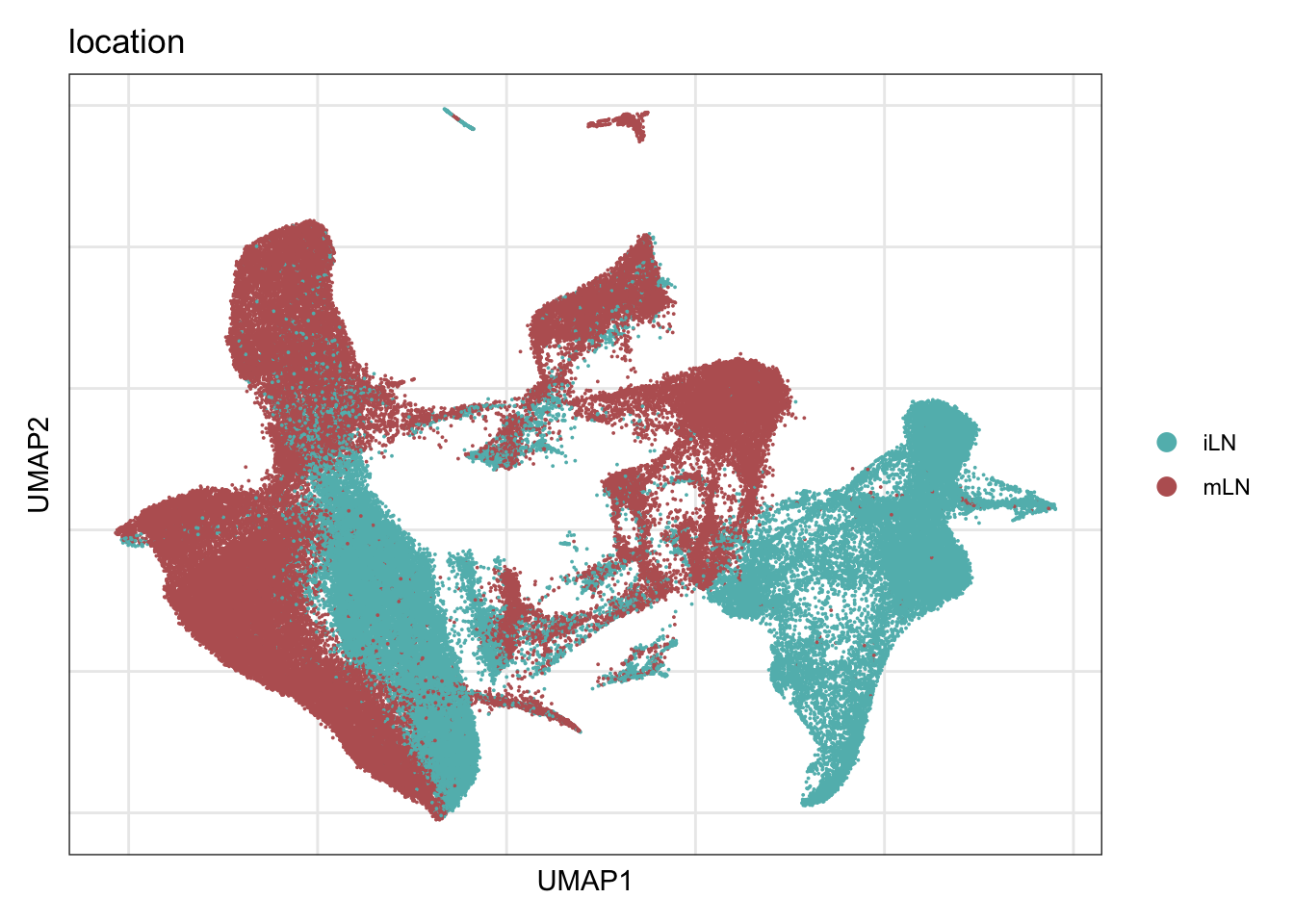
cond
DimPlot(seurat, reduction = "umap", group.by = "cond", cols = colCond,
raster = F, shuffle =T)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")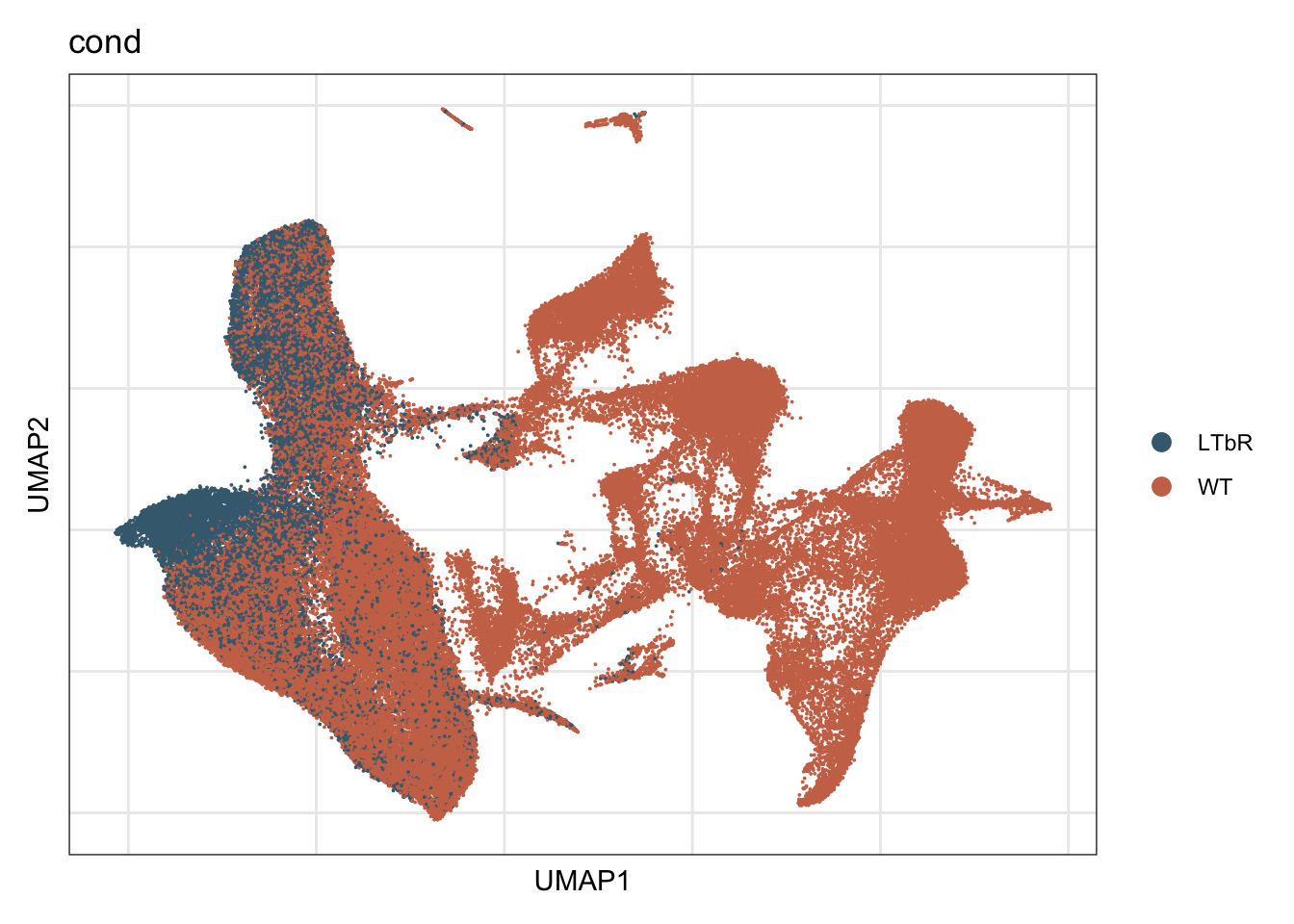
adult WT iLN only
seurat <- subset(seurat, age %in% c("E18", "P7", "3w"), invert=T)
seurat <- subset(seurat, cond %in% c("WT"))
seurat <- subset(seurat, location %in% c("iLN"))
table(seurat$location, seurat$age)
8w E17to7wk
iLN 13827 1133## rerunSeurat
seurat <- NormalizeData(object = seurat)
seurat <- FindVariableFeatures(object = seurat)
seurat <- ScaleData(object = seurat, verbose = FALSE)
seurat <- RunPCA(object = seurat, npcs = 30, verbose = FALSE)
seurat <- RunTSNE(object = seurat, reduction = "pca", dims = 1:20)
seurat <- RunUMAP(object = seurat, reduction = "pca", dims = 1:20)
seurat <- FindNeighbors(object = seurat, reduction = "pca", dims = 1:20)
res <- c(0.8,0.6,0.25,0.4)
for (i in 1:length(res)) {
seurat <- FindClusters(object = seurat, resolution = res[i],
random.seed = 1234)
}Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 14960
Number of edges: 467228
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8130
Number of communities: 19
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 14960
Number of edges: 467228
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8338
Number of communities: 18
Elapsed time: 2 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 14960
Number of edges: 467228
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8893
Number of communities: 13
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 14960
Number of edges: 467228
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8610
Number of communities: 16
Elapsed time: 1 secondscolPal <- c("#DAF7A6", "#FFC300", "#FF5733", "#C70039", "#900C3F", "#b66e8d",
"#61a4ba", "#6178ba", "#54a87f", "#5468a8", "#25328a", "#b6856e",
"#ba6161", "#20714a", "#0073C2FF", "#EFC000FF", "#868686FF",
"#CD534CFF","#7AA6DCFF", "#003C67FF", "#8F7700FF", "#3B3B3BFF",
"#A73030FF", "#4A6990FF")[1:length(unique(seurat$RNA_snn_res.0.4))]
names(colPal) <- unique(seurat$RNA_snn_res.0.4)clustering
DimPlot(seurat, reduction = "umap", group.by = "RNA_snn_res.0.4",
cols = colPal)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")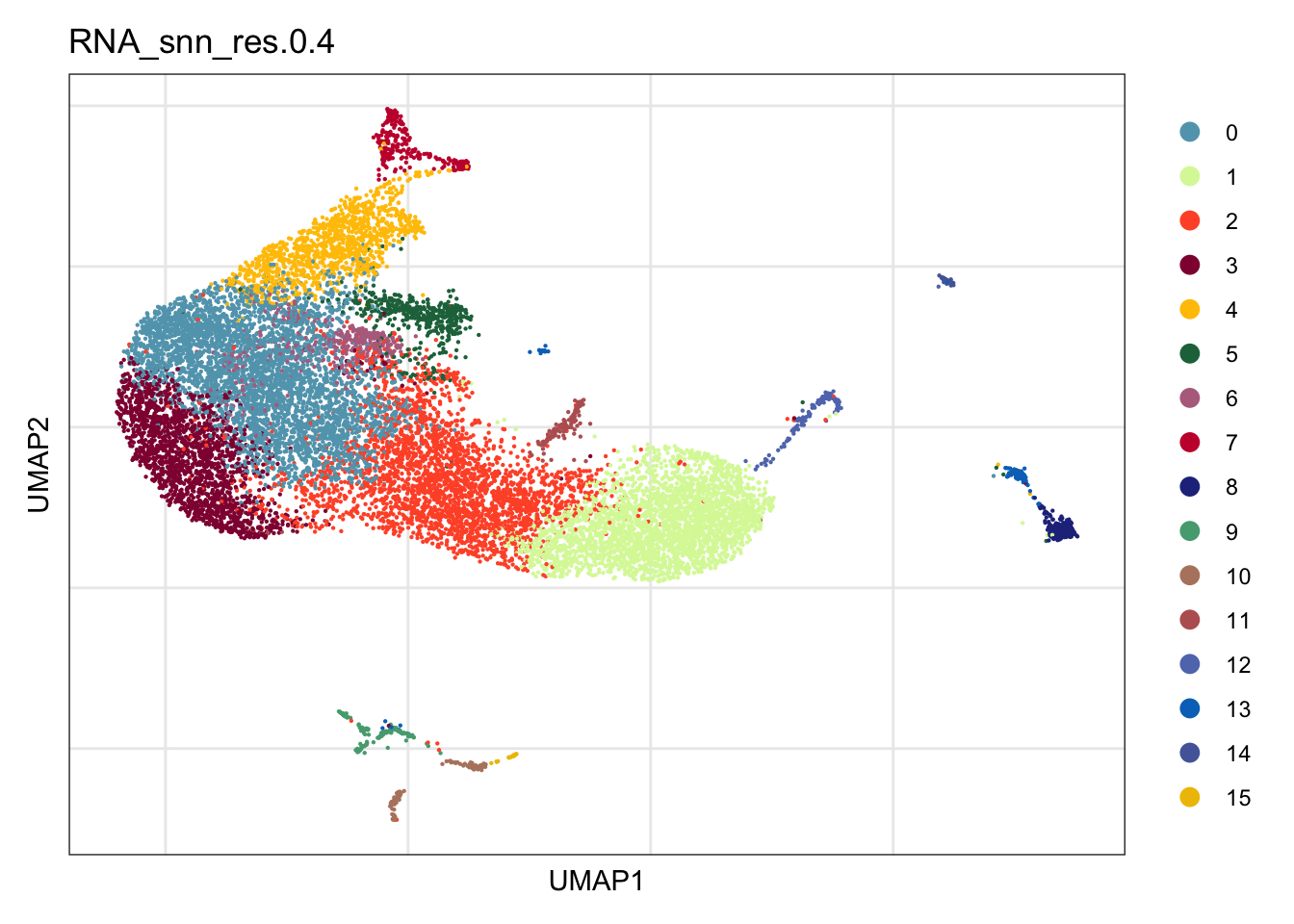
timepoint
DimPlot(seurat, reduction = "umap", group.by = "age", cols = colAge)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")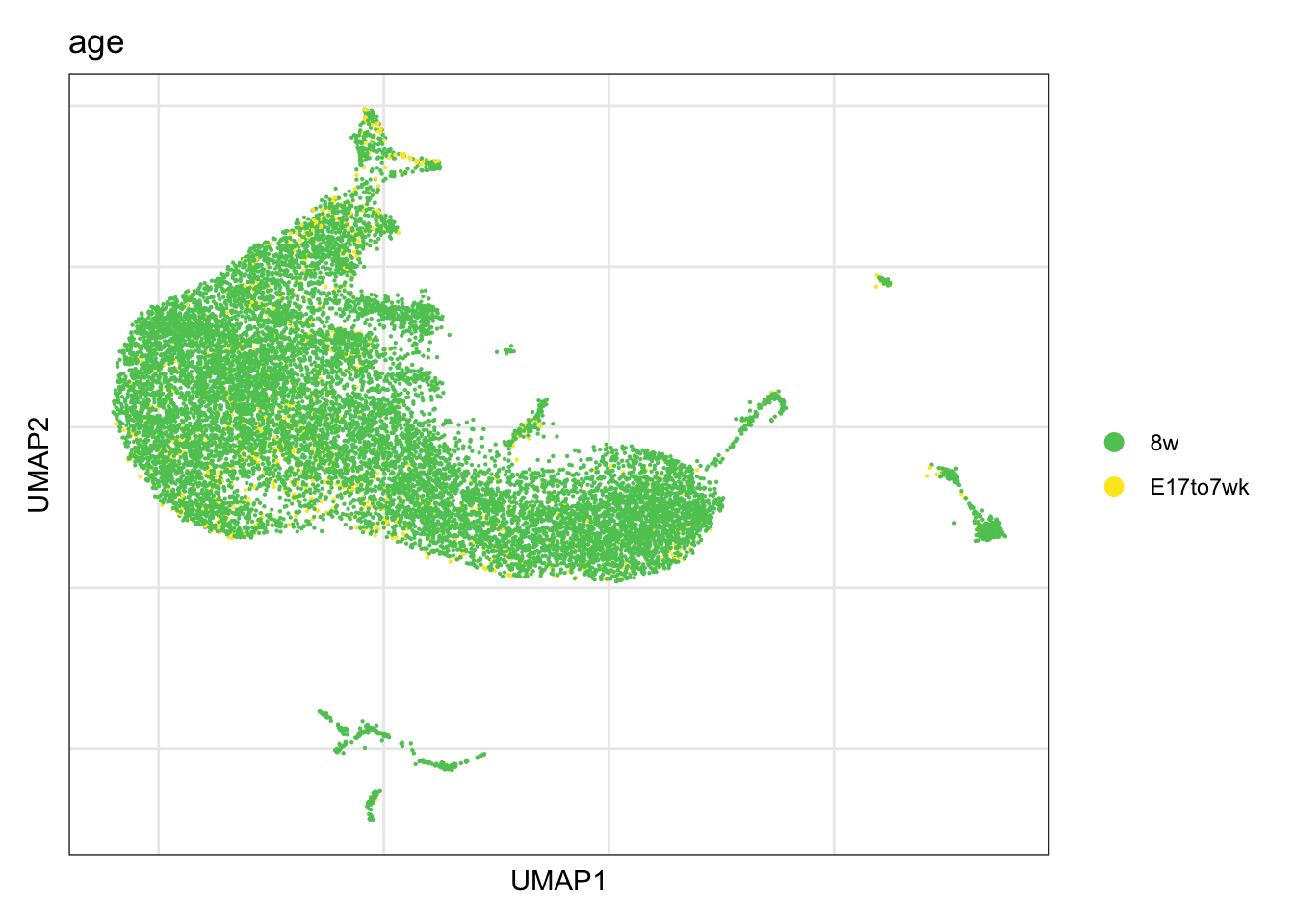
cond
DimPlot(seurat, reduction = "umap", group.by = "cond", cols = colCond)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")
dataset
DimPlot(seurat, reduction = "umap", group.by = "dataset",
cols = colDat)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")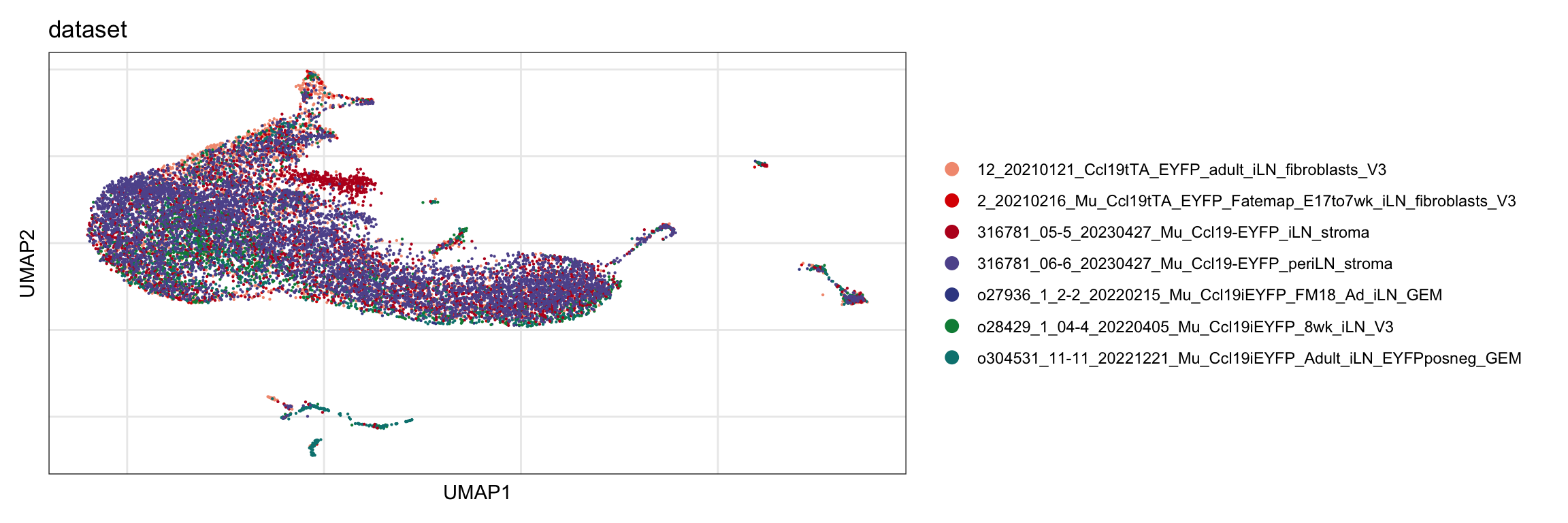
location
DimPlot(seurat, reduction = "umap", group.by = "location", cols = colLoc)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")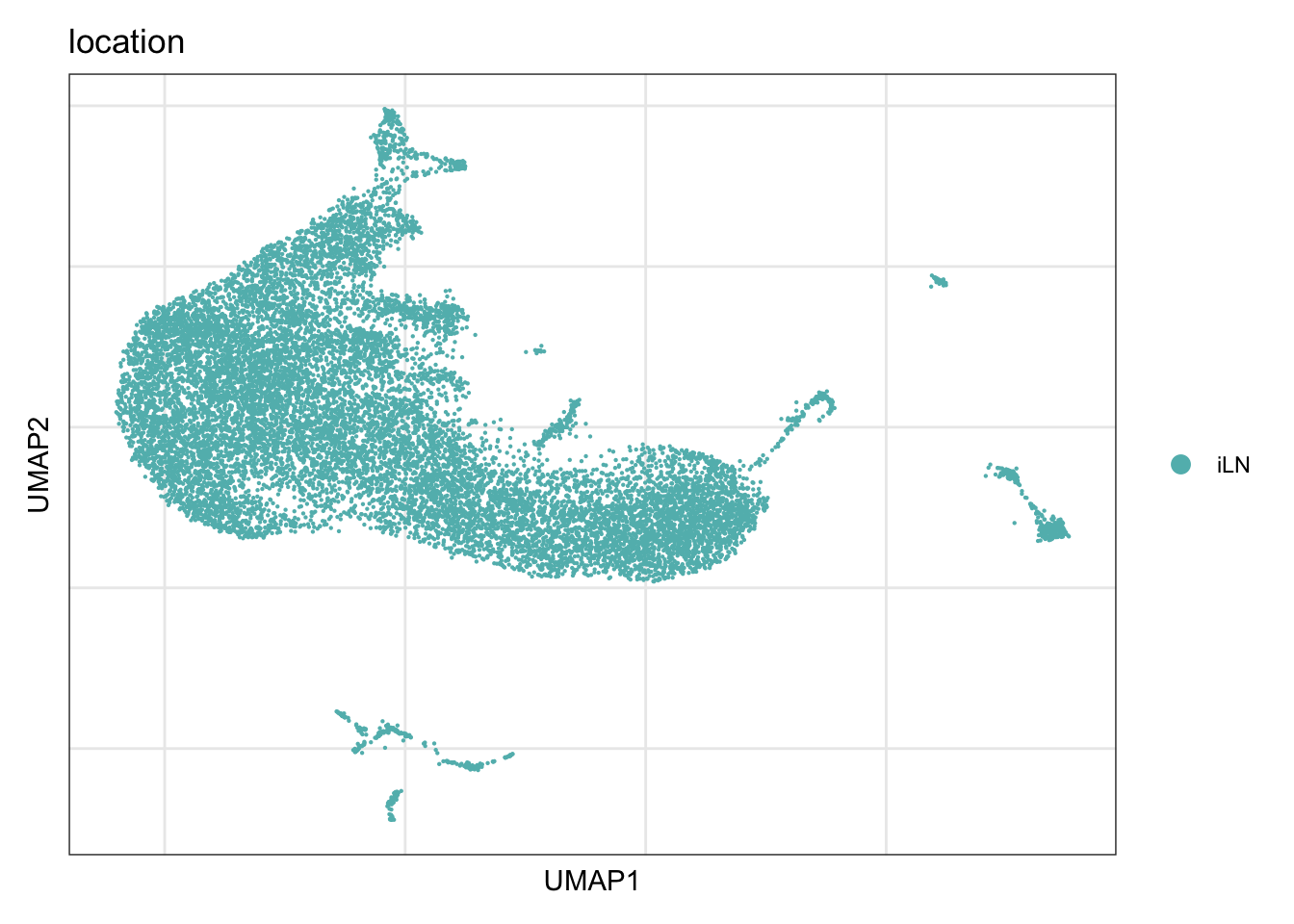
cluster characterization
Idents(seurat) <- seurat$RNA_snn_res.0.4
seurat_markers <- FindAllMarkers(seurat, only.pos = T, logfc.threshold = 0.25)
### plot DE genes top 10 avg logFC
markerAll <- seurat_markers %>% group_by(cluster) %>%
mutate(geneID = gene) %>% top_n(10, avg_log2FC) %>%
mutate(gene=gsub(".*\\.", "", geneID)) %>%
filter(nchar(gene)>1)
grpCnt <- markerAll %>% group_by(cluster) %>% summarise(cnt=n())
gapR <- data.frame(cluster=unique(markerAll$cluster)) %>%
left_join(.,grpCnt, by="cluster") %>% mutate(cumSum=cumsum(cnt))
ordVec <- levels(seurat)
pOut <- avgHeatmap(seurat = seurat, selGenes = markerAll,
colVecIdent = colPal,
ordVec=ordVec,
gapVecR=gapR$cumSum, gapVecC=NULL,cc=T,
cr=F, condCol=F)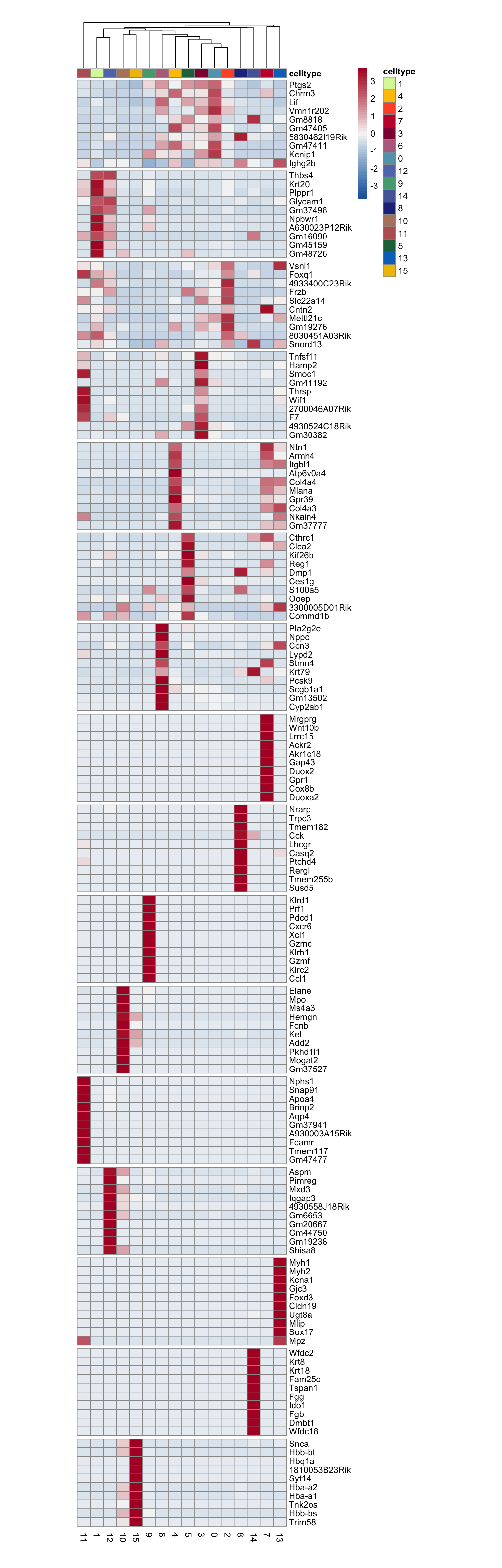
seurat$cluster_plus_loc <- paste0(seurat$RNA_snn_res.0.25, "_",
seurat$location)
Idents(seurat) <- seurat$cluster_plus_loc
ordVec <- sort(levels(seurat))
pOut <- avgHeatmap(seurat = seurat, selGenes = markerAll,
colVecIdent = colPal,
ordVec=ordVec,
gapVecR=gapR$cumSum, gapVecC=NULL,cc=F,
cr=F, condCol=T, colVecCond = colLoc)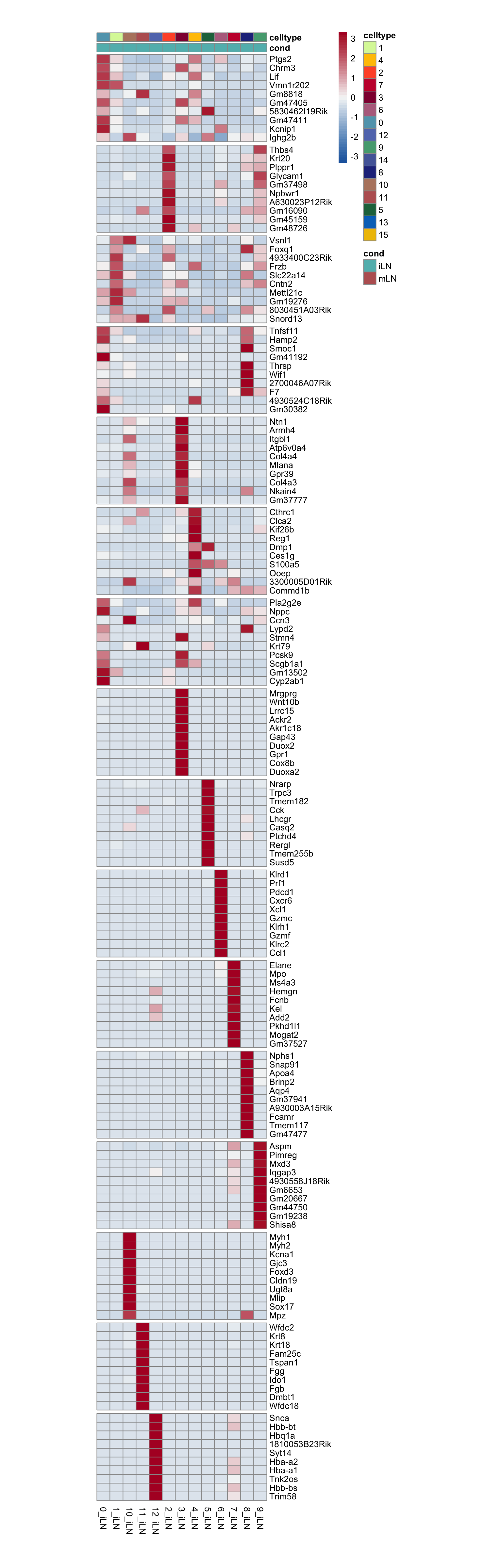
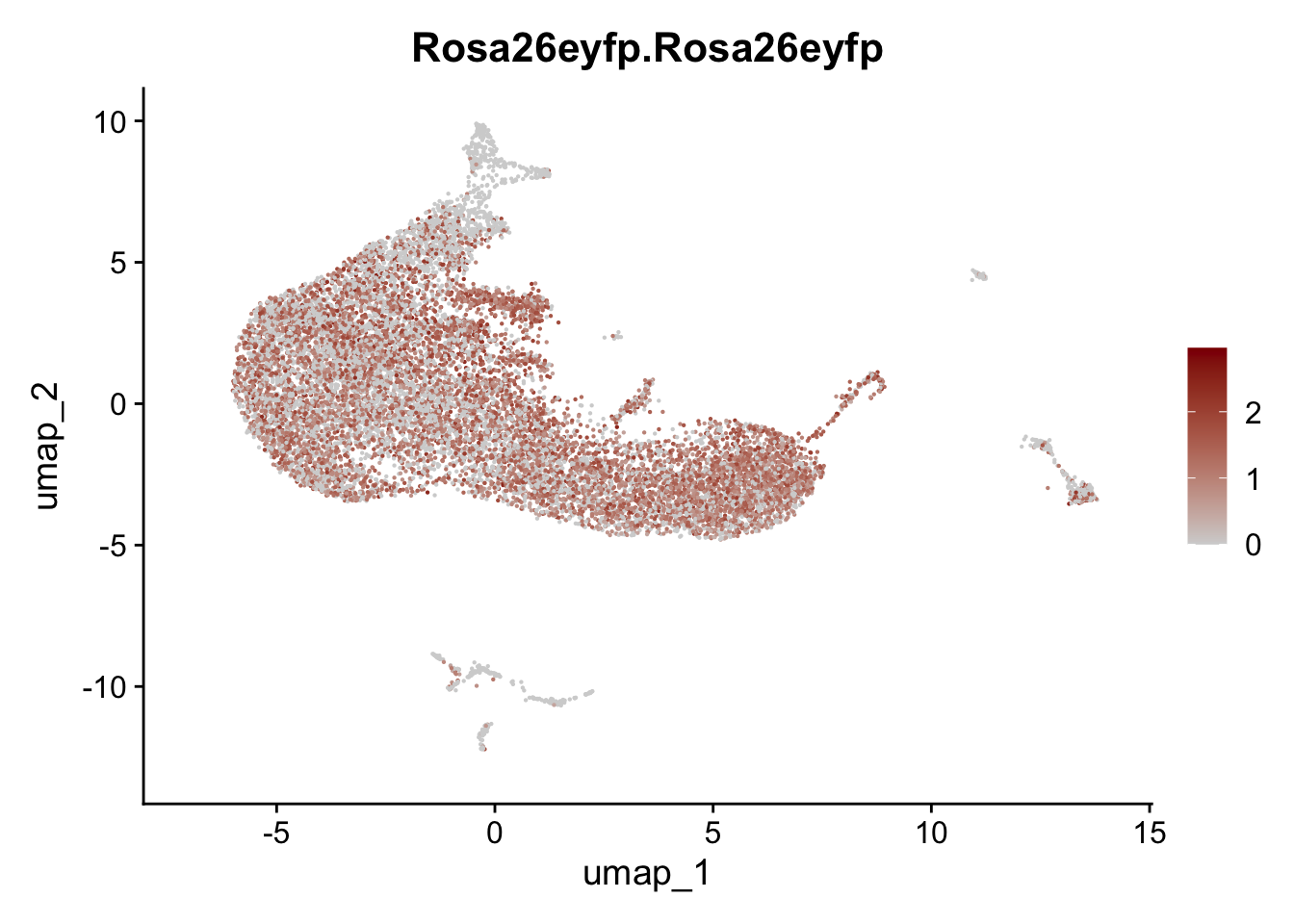
vis selected stroma marker
genes <- data.frame(gene=rownames(seurat)) %>%
mutate(geneID=gsub("^.*\\.", "", gene))
selGenesAll <- data.frame(geneID=c("Rosa26eyfp", "Ccl19", "Ccl21a", "Des",
"Icam1", "Vcam1", "Cnn1", "Acta2", "Rgs5",
"Cox4i2", "Pi16", "Cd34")) %>%
left_join(., genes, by = "geneID")
pList <- sapply(selGenesAll$gene, function(x){
p <- FeaturePlot(seurat, reduction = "umap",
features = x,
cols=c("lightgrey", "darkred"),
order = F)+
theme(legend.position="right")
plot(p)
})
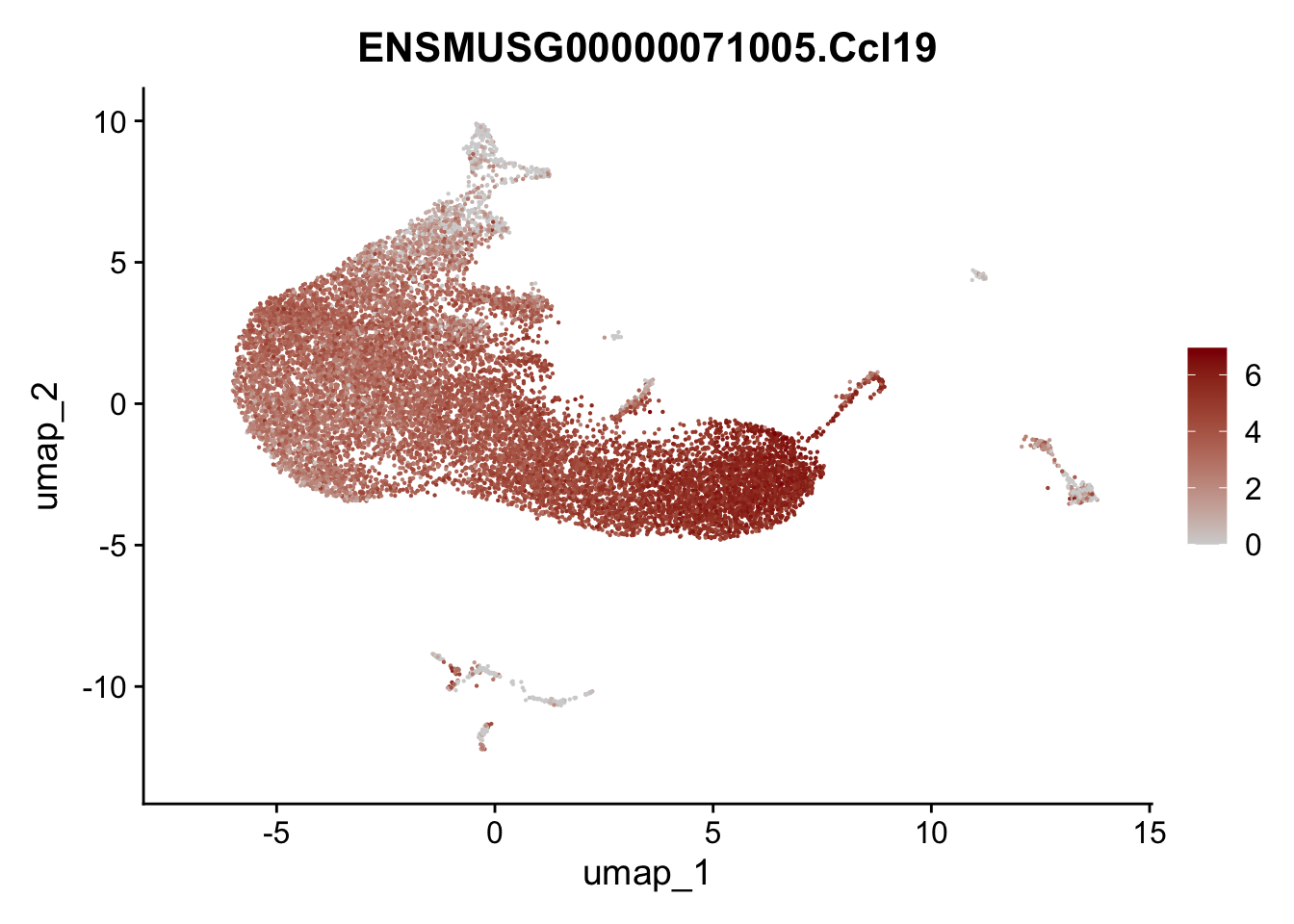
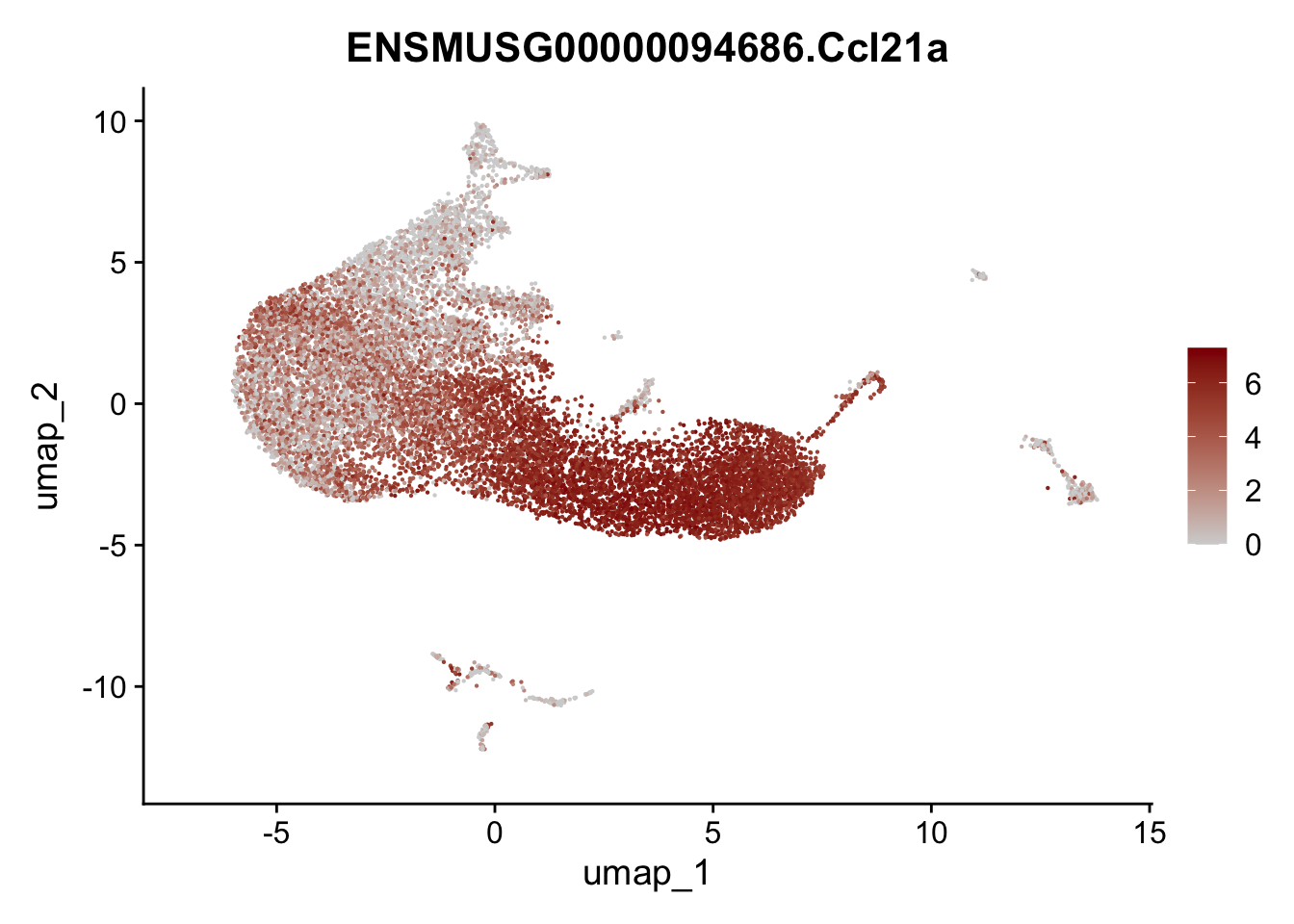
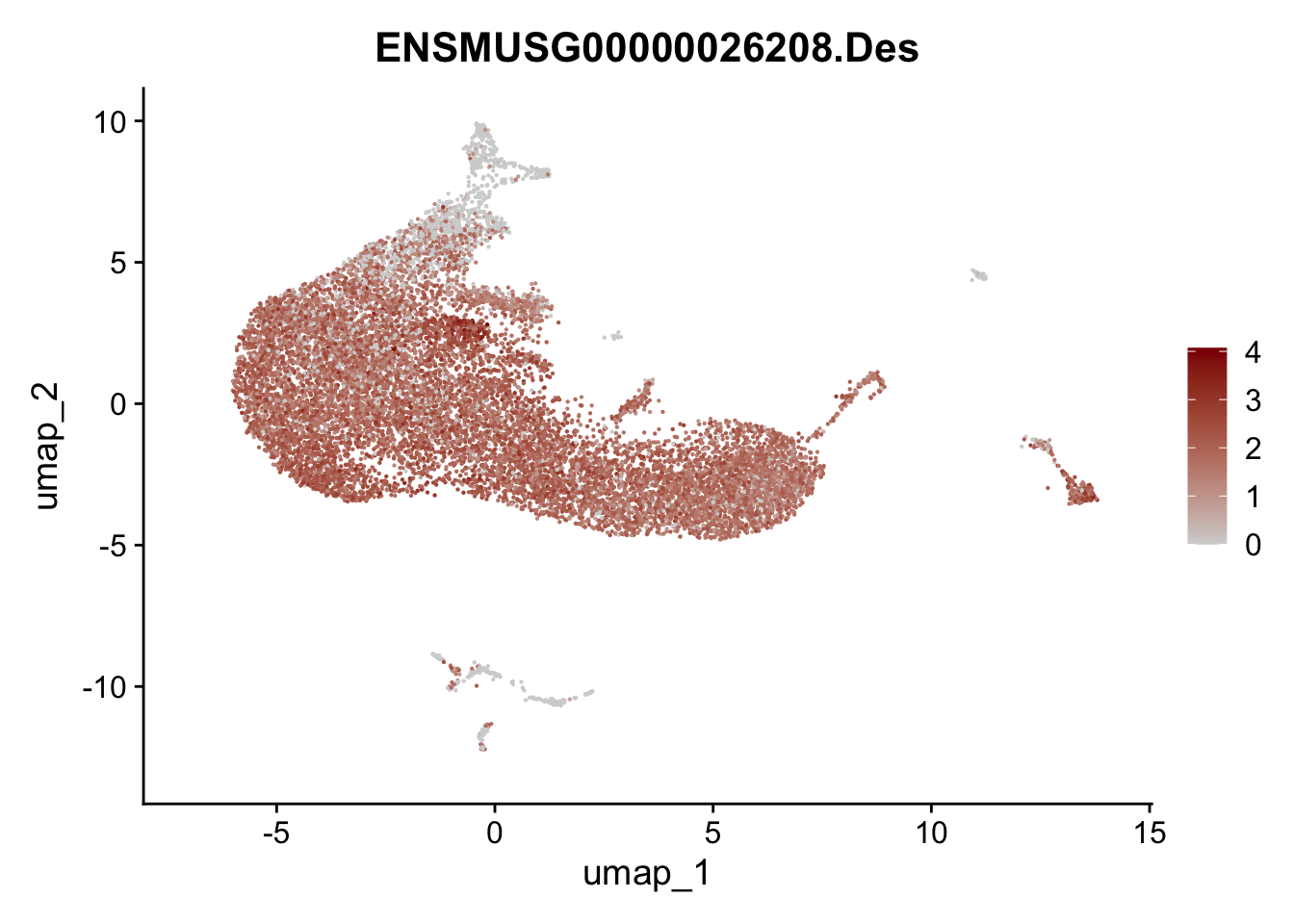

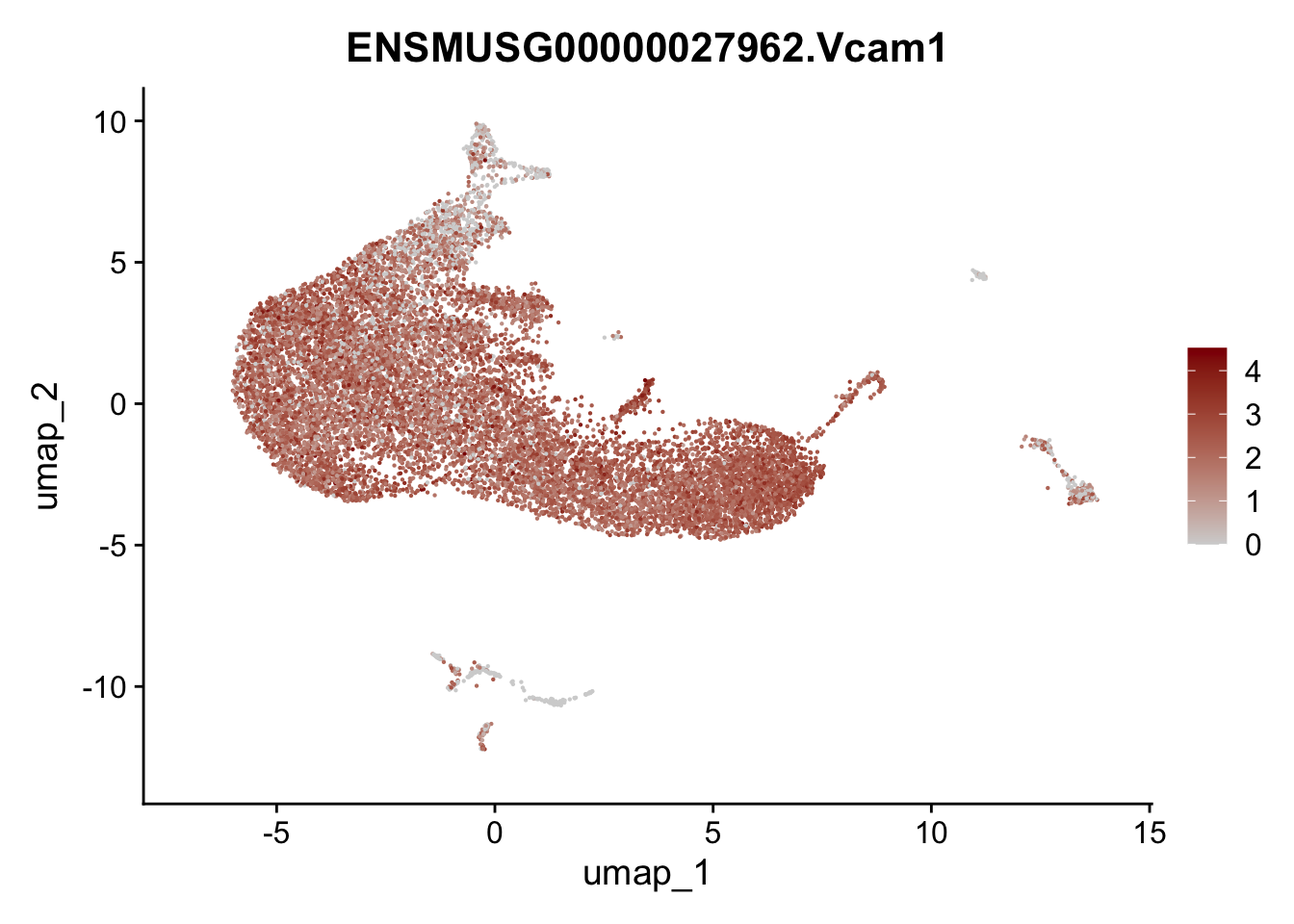
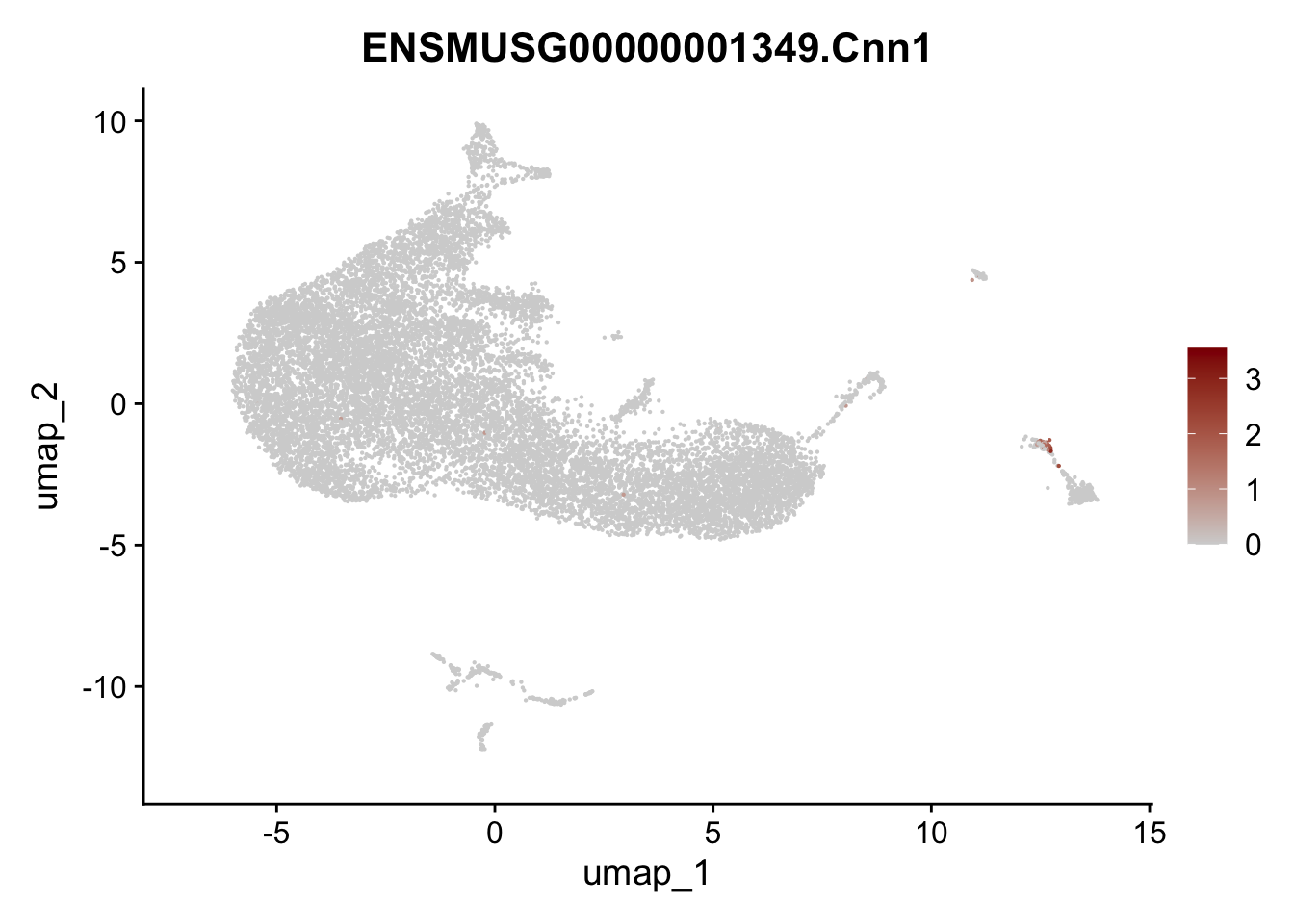
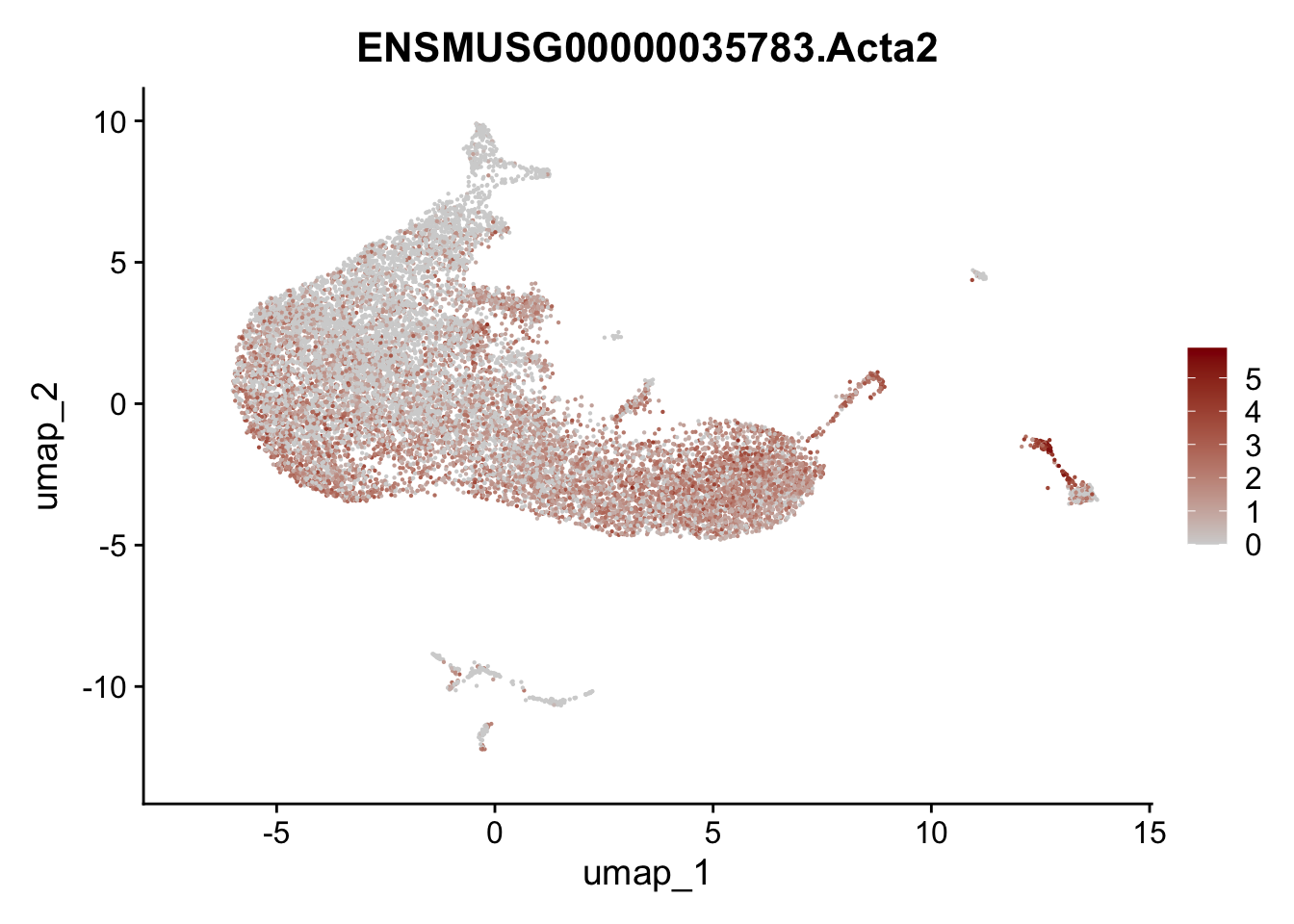
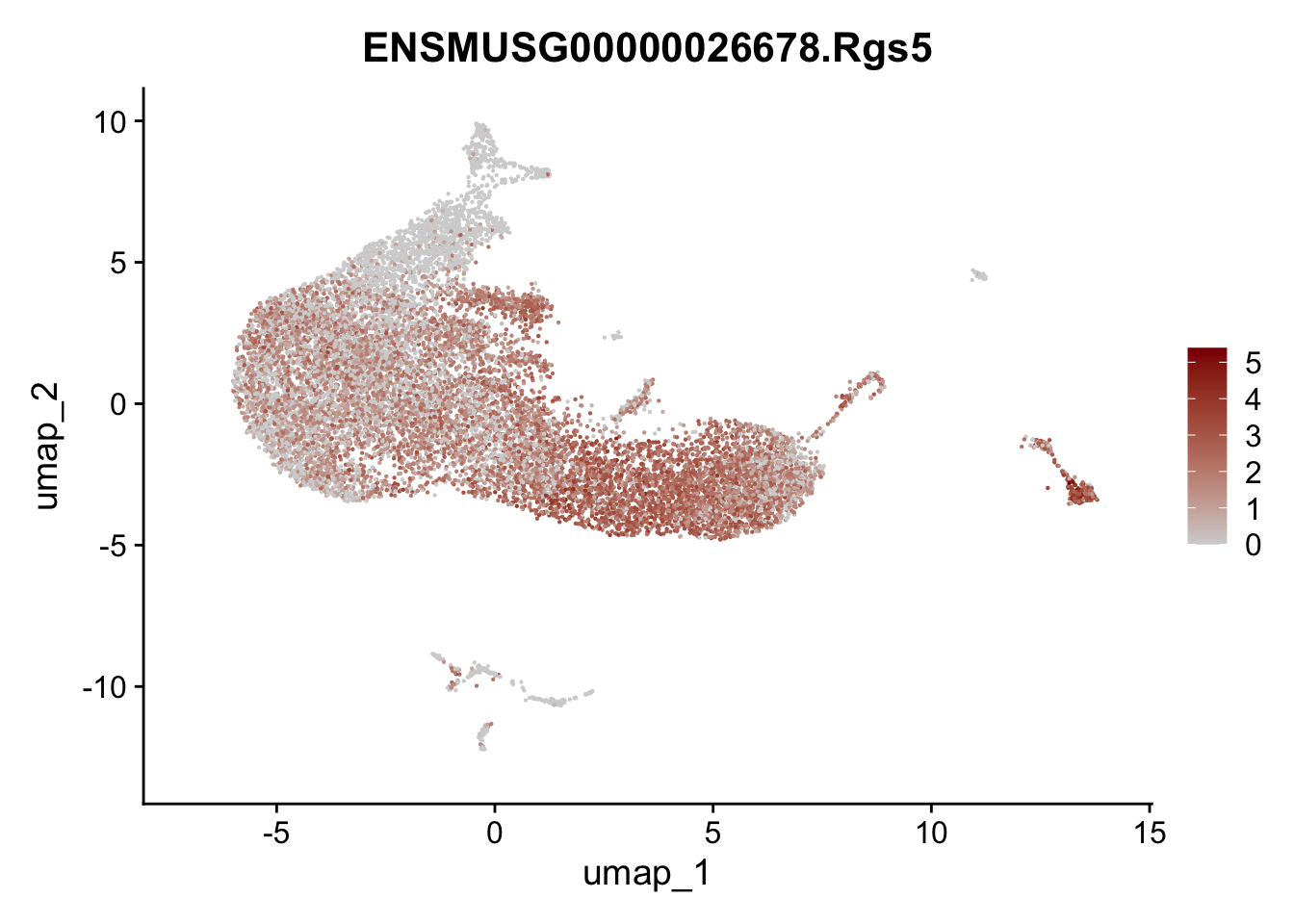
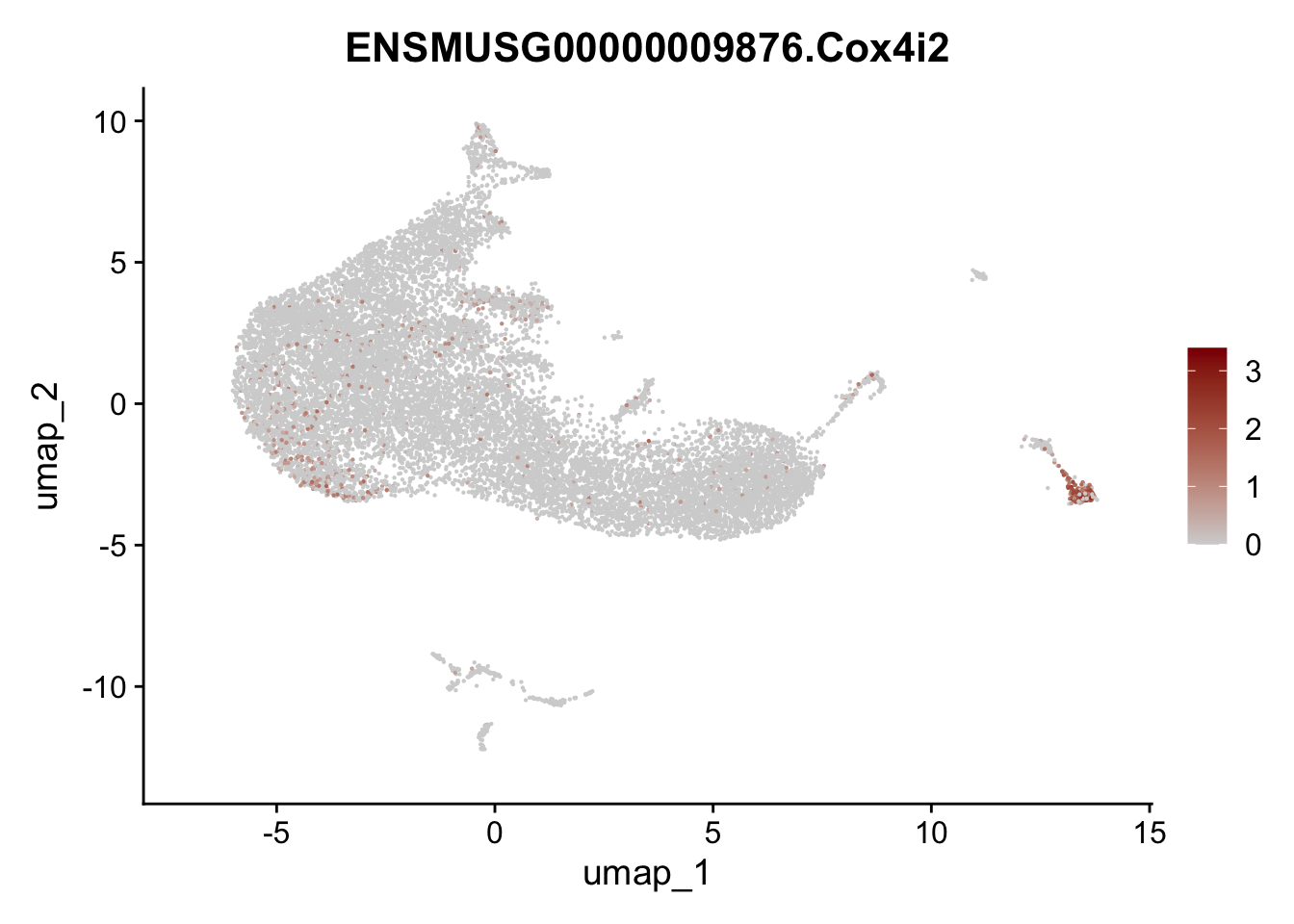
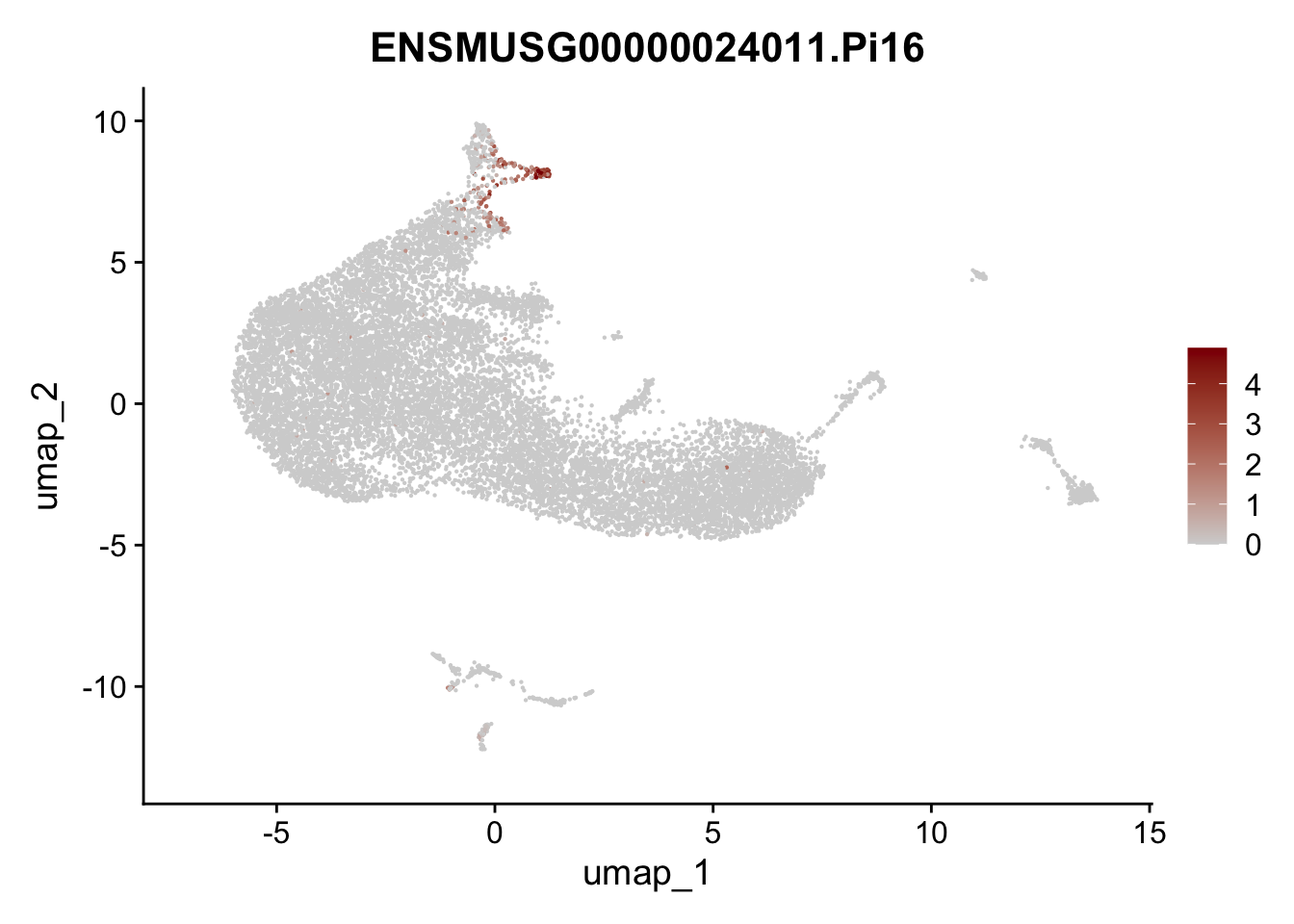
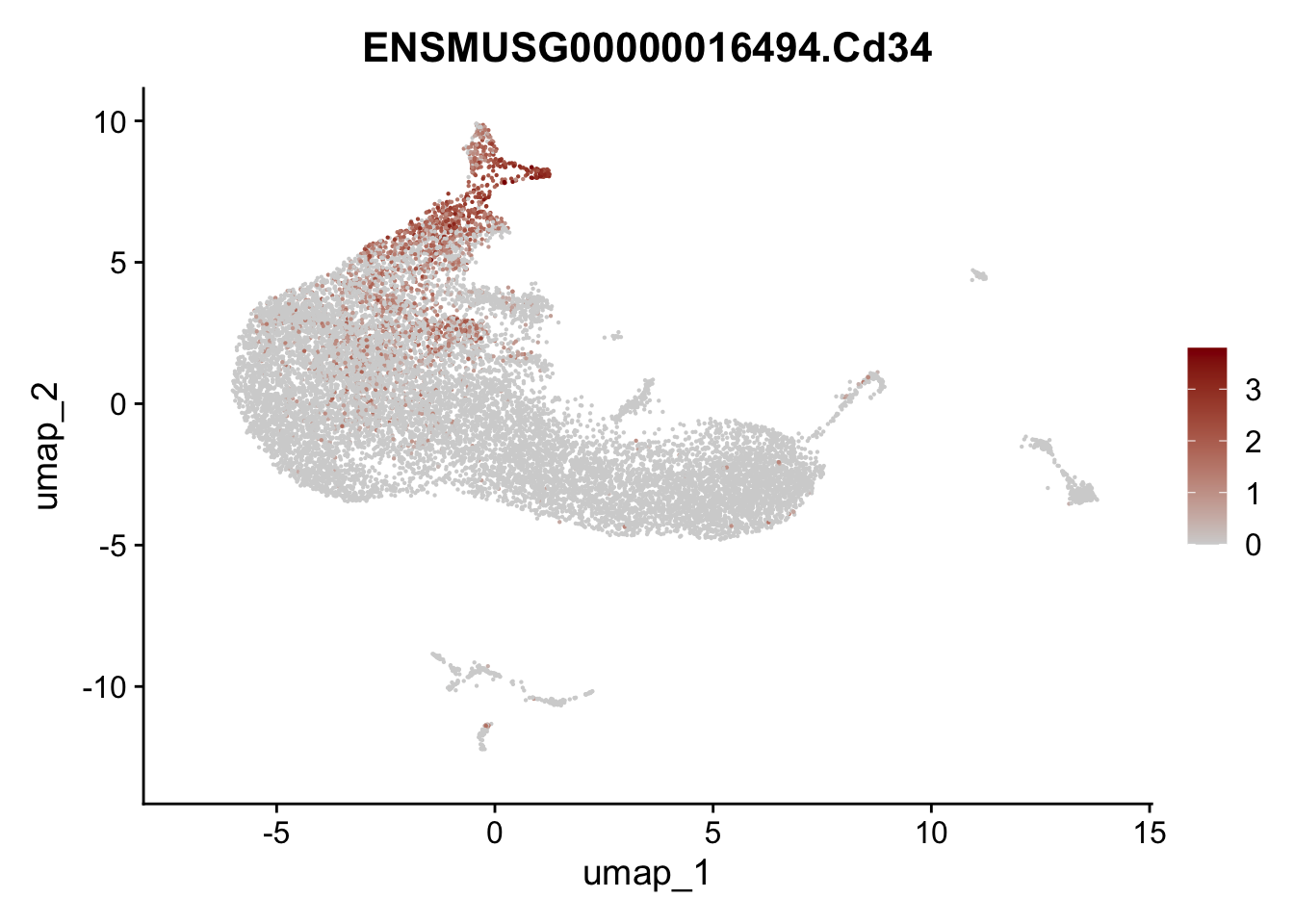
save seurat
saveRDS(seurat, file = paste0(basedir,
"/data/WT_adultOnly_iLNonly_seurat.rds"))
write.table(seurat_markers, quote=F, row.names = T, col.names = T, sep= "\t",
file = paste0(basedir,
"/data/WT_adultOnly_iLNonly_markerGenes.txt"))session info
sessionInfo()R version 4.3.0 (2023-04-21)
Platform: x86_64-apple-darwin20 (64-bit)
Running under: macOS Ventura 13.4.1
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-x86_64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-x86_64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: Europe/Berlin
tzcode source: internal
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] ggsci_3.0.1 scran_1.28.2
[3] scater_1.28.0 scuttle_1.10.3
[5] pheatmap_1.0.12 RColorBrewer_1.1-3
[7] SingleCellExperiment_1.22.0 SummarizedExperiment_1.30.2
[9] Biobase_2.60.0 GenomicRanges_1.52.1
[11] GenomeInfoDb_1.36.4 IRanges_2.36.0
[13] S4Vectors_0.40.1 BiocGenerics_0.48.0
[15] MatrixGenerics_1.12.3 matrixStats_1.2.0
[17] runSeurat3_0.1.0 here_1.0.1
[19] magrittr_2.0.3 Seurat_5.0.2
[21] SeuratObject_5.0.1 sp_2.1-3
[23] lubridate_1.9.3 forcats_1.0.0
[25] stringr_1.5.1 dplyr_1.1.4
[27] purrr_1.0.2 readr_2.1.5
[29] tidyr_1.3.1 tibble_3.2.1
[31] ggplot2_3.5.0 tidyverse_2.0.0
loaded via a namespace (and not attached):
[1] RcppAnnoy_0.0.22 splines_4.3.0
[3] later_1.3.2 bitops_1.0-7
[5] polyclip_1.10-6 fastDummies_1.7.3
[7] lifecycle_1.0.4 edgeR_3.42.4
[9] rprojroot_2.0.4 globals_0.16.2
[11] lattice_0.22-5 MASS_7.3-60.0.1
[13] limma_3.56.2 plotly_4.10.4
[15] rmarkdown_2.26 yaml_2.3.8
[17] metapod_1.8.0 httpuv_1.6.14
[19] sctransform_0.4.1 spam_2.10-0
[21] spatstat.sparse_3.0-3 reticulate_1.35.0
[23] cowplot_1.1.3 pbapply_1.7-2
[25] abind_1.4-5 zlibbioc_1.46.0
[27] Rtsne_0.17 presto_1.0.0
[29] RCurl_1.98-1.14 GenomeInfoDbData_1.2.10
[31] ggrepel_0.9.5 irlba_2.3.5.1
[33] listenv_0.9.1 spatstat.utils_3.0-4
[35] goftest_1.2-3 RSpectra_0.16-1
[37] dqrng_0.3.2 spatstat.random_3.2-3
[39] fitdistrplus_1.1-11 parallelly_1.37.1
[41] DelayedMatrixStats_1.22.6 leiden_0.4.3.1
[43] codetools_0.2-19 DelayedArray_0.26.7
[45] tidyselect_1.2.0 farver_2.1.1
[47] viridis_0.6.5 ScaledMatrix_1.8.1
[49] spatstat.explore_3.2-6 jsonlite_1.8.8
[51] BiocNeighbors_1.18.0 ellipsis_0.3.2
[53] progressr_0.14.0 ggridges_0.5.6
[55] survival_3.5-8 tools_4.3.0
[57] ica_1.0-3 Rcpp_1.0.12
[59] glue_1.7.0 gridExtra_2.3
[61] xfun_0.42 withr_3.0.0
[63] fastmap_1.1.1 bluster_1.10.0
[65] fansi_1.0.6 digest_0.6.34
[67] rsvd_1.0.5 timechange_0.3.0
[69] R6_2.5.1 mime_0.12
[71] colorspace_2.1-0 scattermore_1.2
[73] tensor_1.5 spatstat.data_3.0-4
[75] utf8_1.2.4 generics_0.1.3
[77] data.table_1.15.2 httr_1.4.7
[79] htmlwidgets_1.6.4 S4Arrays_1.0.6
[81] uwot_0.1.16 pkgconfig_2.0.3
[83] gtable_0.3.4 lmtest_0.9-40
[85] XVector_0.40.0 htmltools_0.5.7
[87] dotCall64_1.1-1 scales_1.3.0
[89] png_0.1-8 knitr_1.45
[91] rstudioapi_0.15.0 tzdb_0.4.0
[93] reshape2_1.4.4 nlme_3.1-164
[95] zoo_1.8-12 KernSmooth_2.23-22
[97] vipor_0.4.7 parallel_4.3.0
[99] miniUI_0.1.1.1 pillar_1.9.0
[101] grid_4.3.0 vctrs_0.6.5
[103] RANN_2.6.1 promises_1.2.1
[105] BiocSingular_1.16.0 beachmat_2.16.0
[107] xtable_1.8-4 cluster_2.1.6
[109] beeswarm_0.4.0 evaluate_0.23
[111] locfit_1.5-9.9 cli_3.6.2
[113] compiler_4.3.0 rlang_1.1.3
[115] crayon_1.5.2 future.apply_1.11.1
[117] labeling_0.4.3 plyr_1.8.9
[119] ggbeeswarm_0.7.2 stringi_1.8.3
[121] viridisLite_0.4.2 deldir_2.0-4
[123] BiocParallel_1.34.2 munsell_0.5.0
[125] lazyeval_0.2.2 spatstat.geom_3.2-9
[127] Matrix_1.6-5 RcppHNSW_0.6.0
[129] hms_1.1.3 patchwork_1.2.0
[131] sparseMatrixStats_1.12.2 future_1.33.1
[133] statmod_1.5.0 shiny_1.8.0
[135] ROCR_1.0-11 igraph_2.0.2 date()[1] "Wed Apr 3 12:34:17 2024"